Future Horizons:
10-yearhorizon
Safer germline editing blurs boundaries between therapy and prevention
25-yearhorizon
Polygenic editing erodes boundaries between therapy and enhancement
The two likeliest candidates are base and prime editing. Base editing is powerful against point mutations, which account for 80 per cent of human genetic diseases.21 It also enables mitochondrial gene editing, which is harder to achieve with CRISPR.22 Prime editing is also more specific and accurate, and new research continues to enhance its efficiency.23 Research is under way to replicate CRISPR successes in sickle-cell disease and beyond with base and prime editing.24,25,26 Prime editing can target multiple genes at the same time.27,28
Modified adeno-associated viruses (AAVs) can deliver gene editors, although the large quantities required can trigger dangerous immune responses. Efforts are under way to re-engineer AAV to be bigger and evade immune response. One alternative is more efficient lentiviral vectors, or viruses engineered to make them preferentially infect specific cell types, for example neural cells or airway cells.29
Non-viral delivery has become an increasingly viable alternative, thanks to rapid progress in the use of lipid nanoparticles and inorganic nanoparticle-based delivery systems.30,31
Next-generation editors and delivery - Anticipation Scores
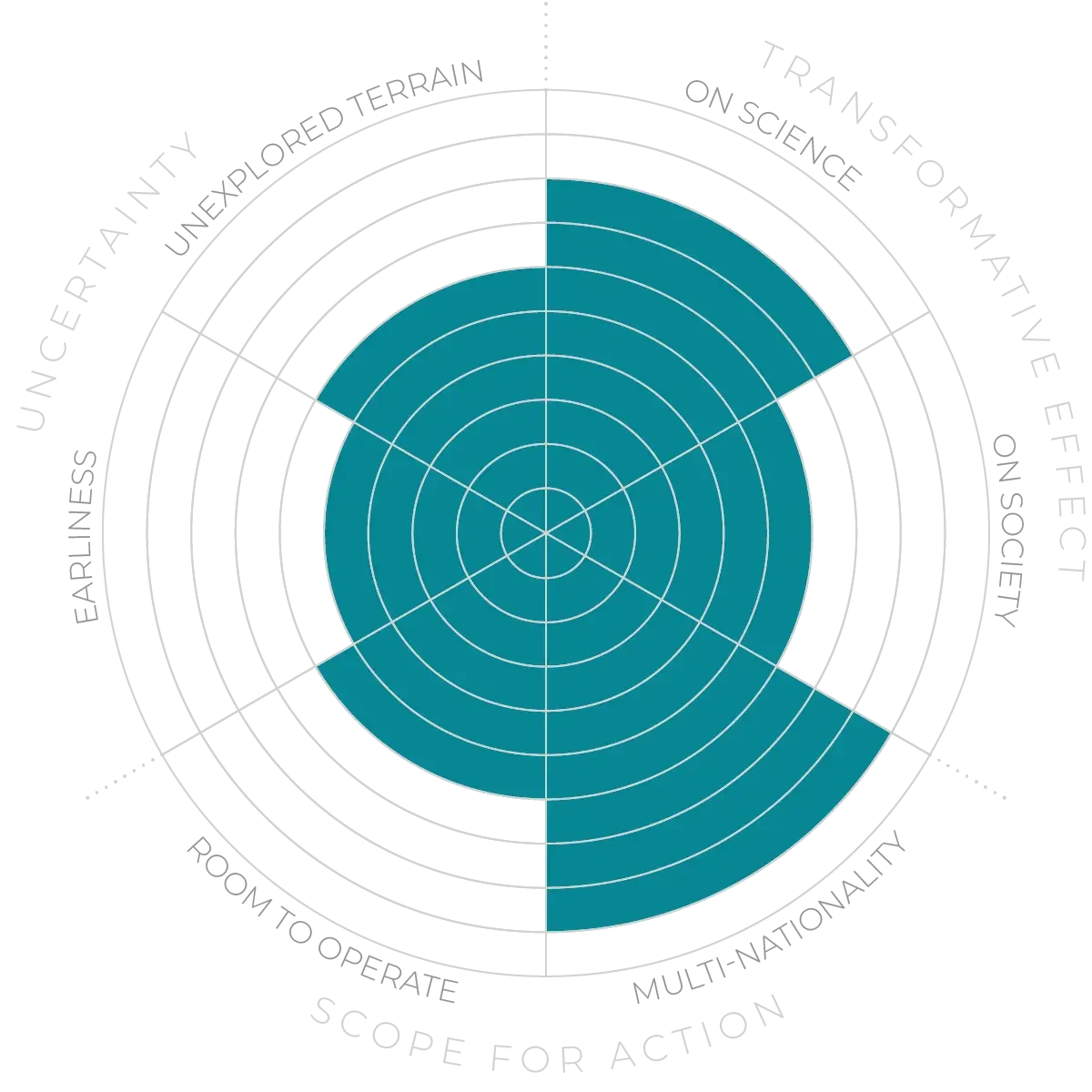
The Anticipation Potential of a research field is determined by the capacity for impactful action in the present, considering possible future transformative breakthroughs in a field over a 25-year outlook. A field with a high Anticipation Potential, therefore, combines the potential range of future transformative possibilities engendered by a research area with a wide field of opportunities for action in the present. We asked researchers in the field to anticipate:
- The uncertainty related to future science breakthroughs in the field
- The transformative effect anticipated breakthroughs may have on research and society
- The scope for action in the present in relation to anticipated breakthroughs.
This chart represents a summary of their responses to each of these elements, which when combined, provide the Anticipation Potential for the topic. See methodology for more information.