Future Horizons:
10-yearhorizon
Genome-reading finds a broad range of applications
25-yearhorizon
Gene-reading goes mainstream
Faster, better and cheaper diagnostics coming into the mainstream will act as a fact-checker on the new generations of genome editors, detecting and preventing DNA-editing errors. These technologies need to be further refined to ensure every laboratory can easily adopt them when in vivo editing becomes mainstream.
Much progress will be thanks to the new ability to do long-read sequencing, more accurate than the previously more common usage of short-read sequencing. This could identify more clinically relevant gene variants and it could also provide epigenetic information that can bring epigenome editing to the clinic.15 Diagnostics will be able to tell patients what kind of gene therapy they are suited for, or even which interventions and lifestyle changes will most affect their chances of expressing a genetic disease. However, analysis and interpretation are a major bottleneck: a shortage of genetic counsellors is an increasing problem.16
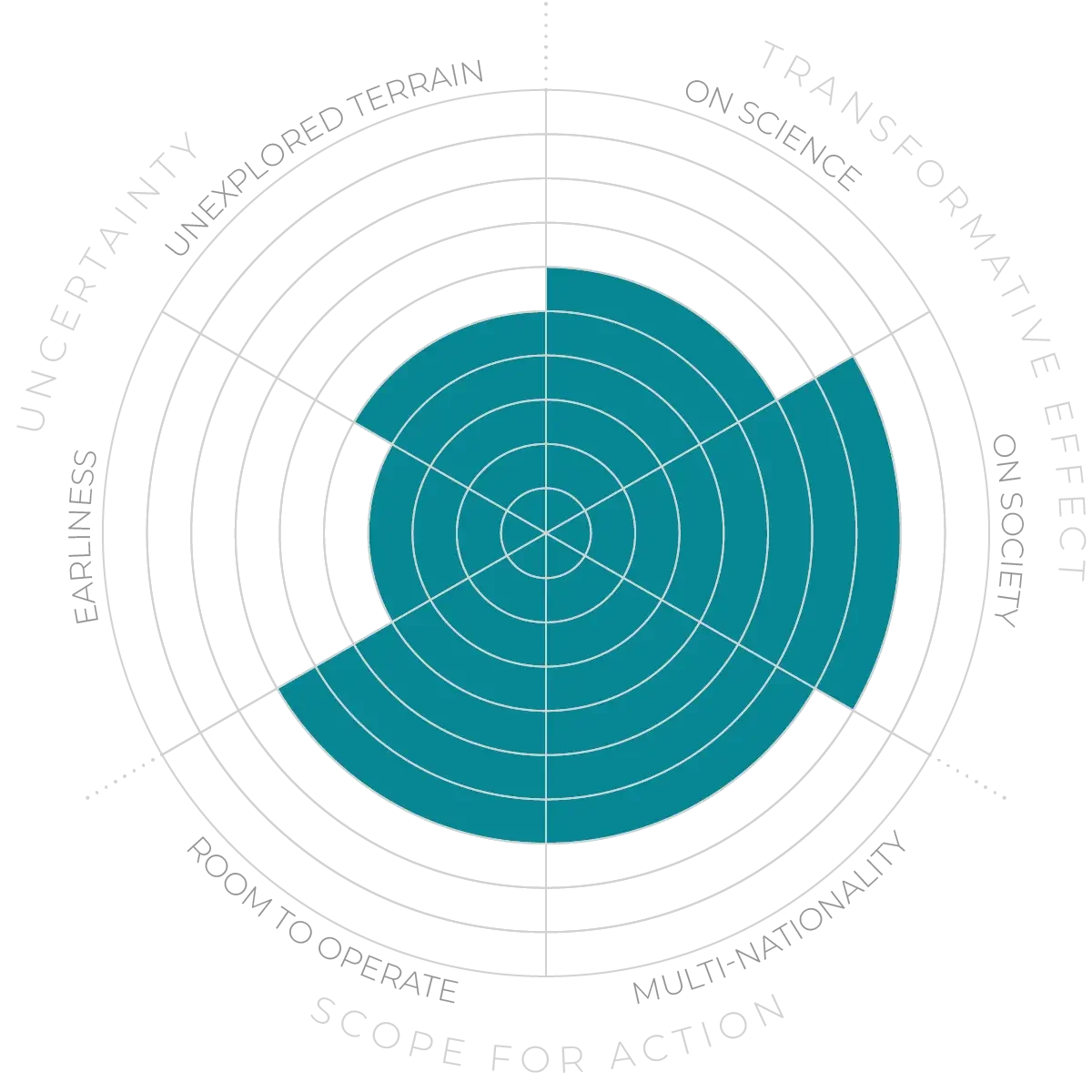
Diagnostics - Anticipation Scores
The Anticipation Potential of a research field is determined by the capacity for impactful action in the present, considering possible future transformative breakthroughs in a field over a 25-year outlook. A field with a high Anticipation Potential, therefore, combines the potential range of future transformative possibilities engendered by a research area with a wide field of opportunities for action in the present. We asked researchers in the field to anticipate:
- The uncertainty related to future science breakthroughs in the field
- The transformative effect anticipated breakthroughs may have on research and society
- The scope for action in the present in relation to anticipated breakthroughs.
This chart represents a summary of their responses to each of these elements, which when combined, provide the Anticipation Potential for the topic. See methodology for more information.