Doing so requires a paradigm shift, however. We will have to switch focus away from the genetic and molecular mechanisms that underpin how individual cells operate, and look at how collective decision-making among groups of cells can lead to larger biological constructs that are greater than the sum of their parts. Fascinatingly, they solve new problems that the parts did not solve, and induce them to behave in ways they would not, by themselves.
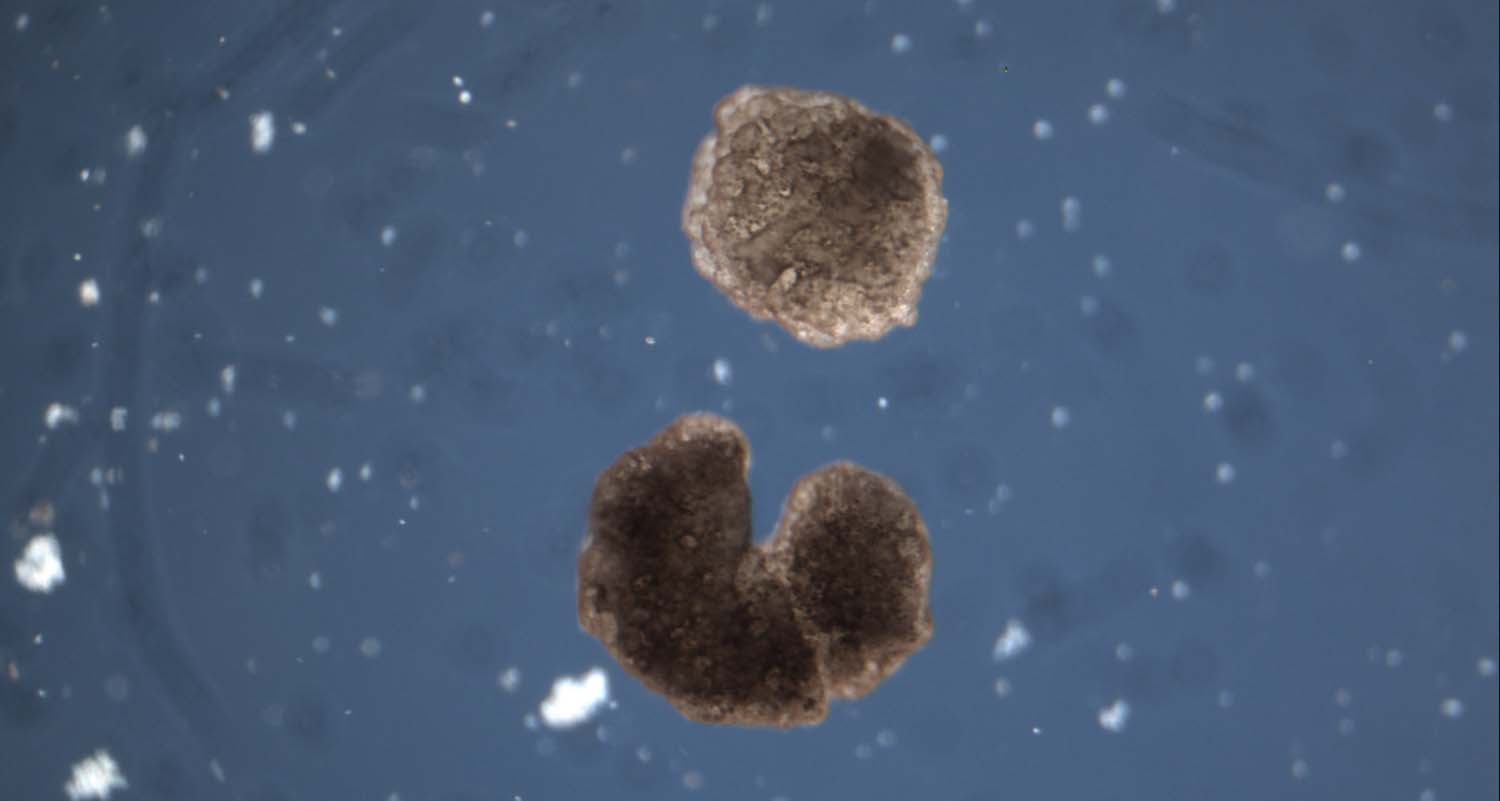
In an effort to gain insights into these processes, our group has investigated whether cells could be coaxed into developing into forms entirely alien to those specified by their genes. One of our members, Douglas Blackiston, harvested skin cells from frog embryos, and used them to create passive structures, while heart cells acted as tiny motors thanks to their regular contractions. These initial designs helped us to understand how the configuration of cells impacted their behaviour, which in turn allowed Josh Bongard and his then PhD student Sam Kriegman to create computer simulations of these synthetic organisms. Bycombining these simulations with evolutionary algorithms that replicate the processes of natural selection, we were able to generate thousands of new designs. The resulting xenobots were able to walk, swim, carry loads and even work together as a swarm to tidy up piles of microscopic debris.
By poking and prodding them into place according to the AI-generated xenobot blueprint, and relying on cells' natural inclination to adhere to each other, we pieced together a variety of simple structures. In 2020, we unveiled our results: a menagerie of synthetic organisms that we dubbed "xenobots", after the African clawed frog (Xenopus laevis) whose cells we used to build them, and the future potential of this system as a bio-robotics platform. Last December, we even showed that our creations could self-replicate by pushing loose cells together to create new Xenobots — a new kind of large-scale replication not known in the natural world.
Today, these tiny machines are still unable to carry out arbitrary practical tasks, and are restricted to simple lab demonstrations that teach us about plasticity, evolution, and engineering with cells that have an unaltered genome. But they represent an entirely new approach to engineering at the smallest scales. Evolution has spent billions of years perfecting tiny machines, far more intricate than anything humans could build today, that boast a vast repertoire of capabilities. Rather than trying to reinvent the wheel, we're learning how to take advantage of their innate strengths and use them as building blocks to create adaptive biological robots.
This requires an entirely different design philosophy. It is less about piecing together carefully engineered parts to micromanage outcomes directly, and more about trying to coordinate what we call “agential materials” that have capabilities and agendas of their own. Crucially, this does not require an exhaustive understanding of the internal mechanisms of the cells themselves — no synthetic biology or genetic engineering was required to create our Xenobots, as we use a kind of behaviour shaping strategy to collaborate with the cells' inherent capacities.
In much the same way as you don't need to understand the neurology of a dog to train it, we are discovering that we can guide the behaviour of cell groups by manipulating their environment and the signals they receive; our on-going efforts will exploit chemical and electrical cues to shift the native collective behaviours of the cells toward desired morphogenetic and behavioural goals.
This project could not have happened without the use of AI to automate our design process. We calculated that actually building all of the designs our evolutionary algorithm explored would have taken 12 million years. Now we are working on automating manufacturing too, which will allow us to carry out the high-throughput experiments that are needed to hone our techniques.
That will also lay the groundwork for commercialisation. While it is still early days, xenobots are just the beginning of a massive field of computer-designed organisms. The technology is so fundamental that it is difficult to imagine all the possible applications. Living cells have a huge amount of in-built machinery for sensing, communicating and even navigating, all of which could be repurposed for a wide variety of use cases. And, as seen in the brain, cells of all kinds can form networks that perform computation and morphogenesis in ways that would be very difficult to implement bottom-up.
An early application is likely to be biosensors that are designed to detect dangers such as pathogens or gas leaks. These would take advantage of a biological system's ability to pick up signals in real-time and rapidly amplify them. Tiny biological robots also have clear future applications in aqueous environments, where they could autonomously navigate into hard-to-reach places to make measurements or clean up toxins.
Ultimately, these biological robots may be able to carry out these kinds of roles inside the human body: chasing down bacteria, killing cancer cells, helping heal wounds, or cleaning up arthritic knee joints. Stringent clinical testing requirements mean that this is likely many years away, but these devices' innate capabilities and biocompatibility could see them quickly overtake the man-made nanobots many are investigating for medical applications.
At a more foundational level, the approaches we have pioneered will be a powerful tool for deciphering the morphogenetic code. Learning how to design, program and control these xenobots is likely to teach us profound lessons about the way the human body organises itself. This could lead to major breakthroughs in regenerative medicine, potentially allowing us to coax our own cells into repairing birth defects, healing organs or re-growing lost limbs.
Democratising this technology will be key to realising that potential. That is why we have made our research freely available, and open-sourced the software used to design our xenobots. But it is crucial that we start anticipating future developments in this field: the technology's relative simplicity and wide applicability means that it is not likely to remain in the lab for long.
The technology should raise profound philosophical questions too. It seems inevitable that xenobots are likely to blur the lines between machines and organisms, and will start to chip away at notions of human, and even biological, exceptionalism that underpin much of Western philosophy. They will provoke us to ask what it means to be human — is a xenobot made from your own cells part of you?
Designing synthetic organisms not found in nature is also likely to provoke pushback from those concerned about upsetting the natural order of things. But it is important to remember that natural evolution does not optimise for happiness, intelligence or any of the things we value. The modern world is beset with human suffering, animal suffering, and environmental destruction and we are now on the cusp of a technology that could help fix many of these issues. In our view, we have a moral and ethical responsibility to do better than the meandering search process that has governed the development of life on this earth so far, using rational understanding of emergent agency to improve quality of life for all.